The Crook Laboratory at San Francisco State University is located in the Biology Building. Our animals are maintained in semi-outdoor, naturalistic seawater systems, providing sunlight and a natural day/night cycle. In addition to our research animals, we also maintain a large colony of live, planktonic food species.
Lab facilities include artificial seawater systems at three different temperatures (two tropical and one temperate), each with isolated tank zones for in situ behavioral studies. We have daylight, red-light and dark zones for behavior, and automated behavioural analysis software that tracks multiple animals.
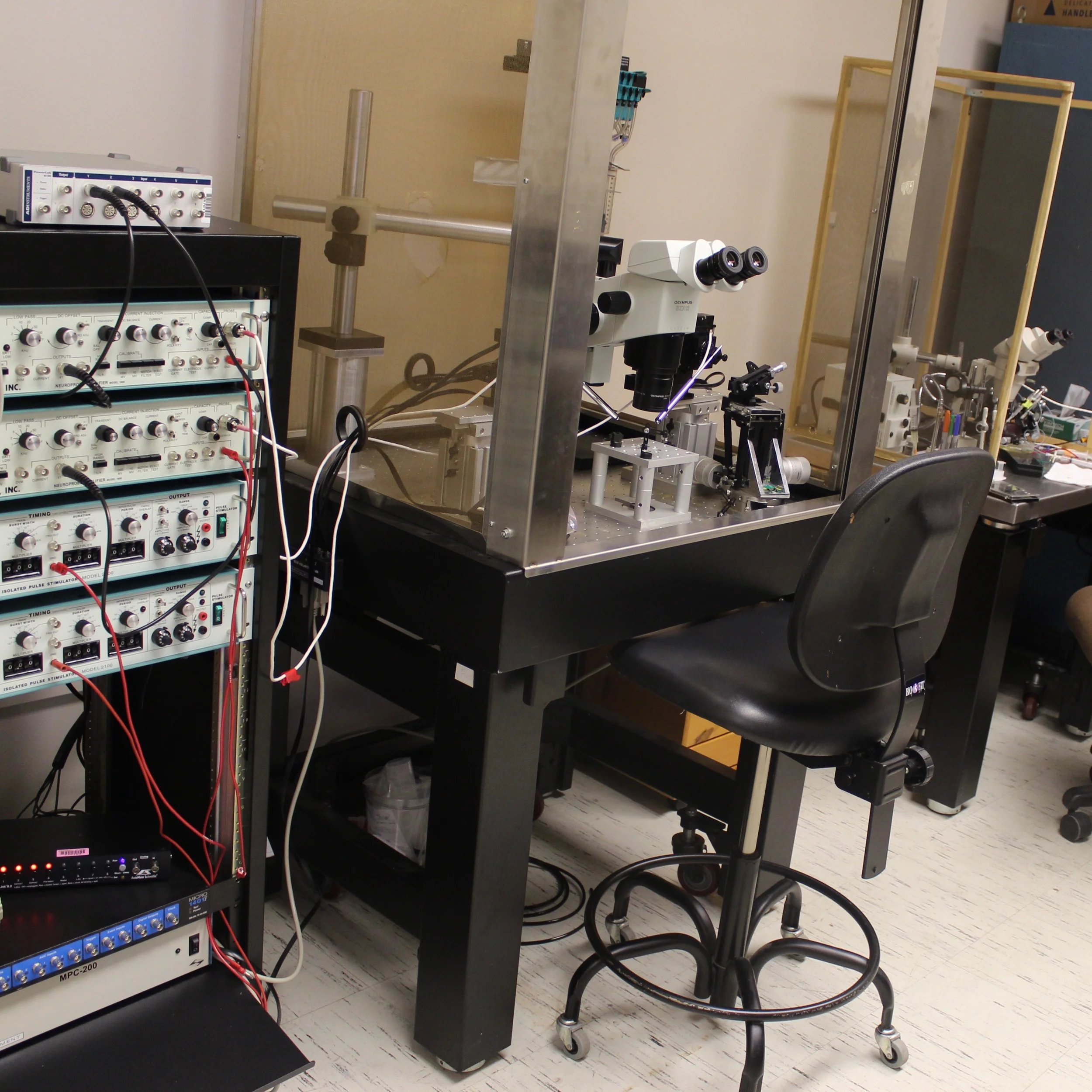
Our electrophysiology facility includes intracellular and extracellular rigs, along with stereoscopes for dissection and tissue preparation. Desk space and computers are available for students to complete video and electrophysiology data analysis, and work on presentations and manuscripts. We also share molecular biology and sectioning equipment with several collaborating labs.
Our facilities
General lab space
Our lab space contains bench space for typical wet lab tasks - making solutions, preparing tissue samples, conducting behavioral studies and performing water quality assessments and measurements. There are work-benches for eight students, along with several workstations for image and video data analysis.
Our dissection area houses a trinocular teaching microscope with live output onto a monitor for training in tissue preparation, dissection and labeling.
Tropical tank room
Our tropical tank room houses our colonies of squid and cuttlefish and also tropical species of octopus in two independent, recirculating seawater systems. Our larger system is 600 US gallons (~2300 liters) and our smaller system is 450 US gallons (1700 liters). Each system is completely self-contained, with independent temperature control and filtration.
Within these systems we also keep our grass shrimp, our mysid breeding colony and a breeding population of copepods. Filtration is provided primarily by live rock and biological filtration media.
Temperate rack system
This recirculating system is housed in the department’s common seawater facility, which supports research and teaching. This 300 gallon rack houses our Octopus bimaculoides sub-adults and juveniles in large enclosures with complex habitats.
Neurophysiology and Microscopy room
This room contains two electrophysiology rigs - one for extracellular recordings, and one for intracellular (sharp electrode) recordings. We also have an automated, fluorescent stereomicroscope with dye-loading capacities, and a large image-analysis workstation for confocal and EM data analysis. Software includes LabChart, Amira, Matlab, FIJI and Reconstruct for microscopy and electrophysiology, Prism and R Studio for statistics, and DeepLabCut and Ethovision for behavioral analysis.
Shared Facilities
We also co-own equipment shared with other laboratories in Hensill Hall, allowing us to conduct projects in collaboration and also expand the range of projects we can undertake.
Our shared equipment includes a Leica cryostat for frozen sections, a vibratome for making slice preparations for calcium imaging, an ultramicrotome for EM sections, a large molecular biology area that supports our HCR and immunolabeling work, and several shared microscopes, including a new Leica Stellaris confocal and a Zeiss 710 confocal.